Desloratadine; Pseudoephedrine Sulfate Patent Expiration
Desloratadine; Pseudoephedrine Sulfate is Used for relieving both nasal and non-nasal symptoms of seasonal allergic rhinitis. It was first introduced by Organon Llc A Sub Of Organon And Co
Desloratadine; Pseudoephedrine Sulfate Patents
Given below is the list of patents protecting Desloratadine; Pseudoephedrine Sulfate, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
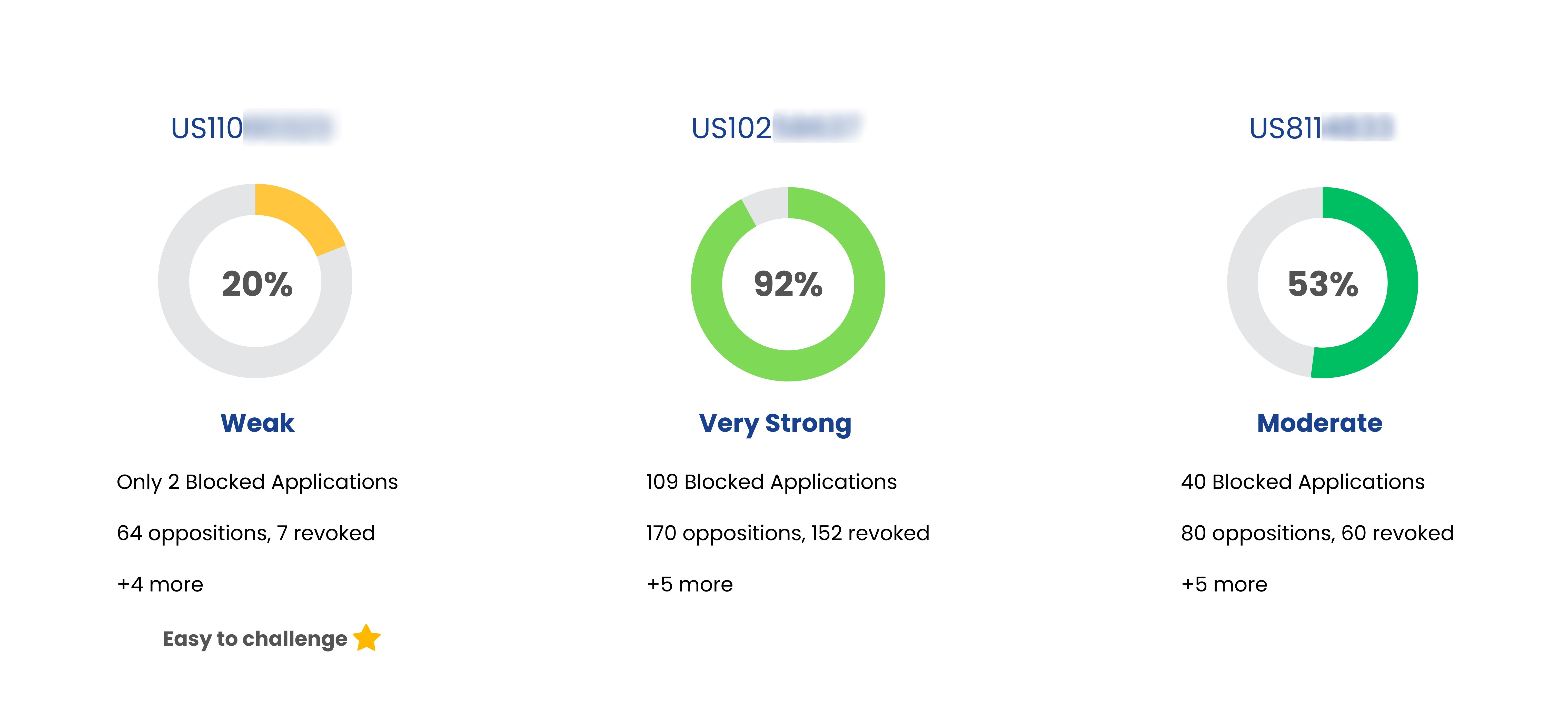
| Clarinex-d 12 Hour | US6100274 | 8-chloro-6,11-dihydro-11- ](4-piperidylidine)-5H-benzo[5,6]cyclohepta[1,2-bpyridine oral compositions |
Jul 07, 2019
(Expired) | Organon Llc |
| Clarinex-d 12 Hour |
US6100274 (Pediatric) | 8-chloro-6,11-dihydro-11- ](4-piperidylidine)-5H-benzo[5,6]cyclohepta[1,2-bpyridine oral compositions |
Jan 07, 2020
(Expired) | Organon Llc |
| Clarinex-d 12 Hour | US6709676 | Extended release oral dosage composition |
Feb 18, 2021
(Expired) | Organon Llc |
| Clarinex-d 12 Hour | US7214683 | Compositions of descarboethoxyloratadine |
Dec 30, 2014
(Expired) | Organon Llc |
| Clarinex-d 12 Hour |
US7214683 (Pediatric) | Compositions of descarboethoxyloratadine |
Jun 30, 2015
(Expired) | Organon Llc |
| Clarinex-d 12 Hour | US7214684 | Methods for the treatment of allergic rhinitis |
Dec 30, 2014
(Expired) | Organon Llc |
| Clarinex-d 12 Hour |
US7214684 (Pediatric) | Methods for the treatment of allergic rhinitis |
Jun 30, 2015
(Expired) | Organon Llc |
| Clarinex-d 12 Hour | US7618649 | Extended release oral dosage composition |
Dec 19, 2020
(Expired) | Organon Llc |
| Clarinex-d 12 Hour |
US7618649 (Pediatric) | Extended release oral dosage composition |
Jun 19, 2021
(Expired) | Organon Llc |
| Clarinex-d 12 Hour | US8187630 | Extended release oral dosage composition |
Dec 19, 2020
(Expired) | Organon Llc |
| Clarinex D 24 Hour | US6100274 | 8-chloro-6,11-dihydro-11- ](4-piperidylidine)-5H-benzo[5,6]cyclohepta[1,2-bpyridine oral compositions |
Jul 07, 2019
(Expired) | Organon |
| Clarinex D 24 Hour |
US6100274 (Pediatric) | 8-chloro-6,11-dihydro-11- ](4-piperidylidine)-5H-benzo[5,6]cyclohepta[1,2-bpyridine oral compositions |
Jan 07, 2020
(Expired) | Organon |
| Clarinex D 24 Hour | US6979463 | Stable extended release oral dosage composition |
Mar 28, 2022
(Expired) | Organon |
| Clarinex D 24 Hour | US7214683 | Compositions of descarboethoxyloratadine |
Dec 30, 2014
(Expired) | Organon |
| Clarinex D 24 Hour |
US7214683 (Pediatric) | Compositions of descarboethoxyloratadine |
Jun 30, 2015
(Expired) | Organon |
| Clarinex D 24 Hour | US7214684 | Methods for the treatment of allergic rhinitis |
Dec 30, 2014
(Expired) | Organon |
| Clarinex D 24 Hour |
US7214684 (Pediatric) | Methods for the treatment of allergic rhinitis |
Jun 30, 2015
(Expired) | Organon |
| Clarinex D 24 Hour | US7618649 | Extended release oral dosage composition |
Dec 19, 2020
(Expired) | Organon |
| Clarinex D 24 Hour |
US7618649 (Pediatric) | Extended release oral dosage composition |
Jun 19, 2021
(Expired) | Organon |
| Clarinex D 24 Hour | US7820199 | Stable extended release oral dosage composition |
Mar 28, 2022
(Expired) | Organon |
| Clarinex D 24 Hour |
US7820199 (Pediatric) | Stable extended release oral dosage composition |
Sep 28, 2022
(Expired) | Organon |
Coming Soon
Patent Strength Analyzer
YesNo
Thank you for your response 🥳
Desloratadine; Pseudoephedrine Sulfate Generics
Only one generic application has been filed for Desloratadine; Pseudoephedrine Sulfate.
Given below is the list of companies who have filed for Desloratadine; Pseudoephedrine Sulfate generic.
1. DR REDDYS LABS LTD
Dr Reddys Laboratories Ltd has filed for 1 generic for Desloratadine; Pseudoephedrine Sulfate. This 5mg;240mg version comes by the name DESLORATADINE AND PSEUDOEPHEDRINE SULFATE 24 HOUR. Given below are the details of the strengths of this generic introduced by Dr Reddys Labs Ltd.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 5MG; 240MG
(reference standard) | tablet, extended release | Prescription | ORAL | N/A | Apr 26, 2011 |
