Sofosbuvir; Velpatasvir Patent Expiration
Sofosbuvir; Velpatasvir is Used for treating hepatitis C. It was first introduced by Gilead Sciences Inc
Sofosbuvir; Velpatasvir Patents
Given below is the list of patents protecting Sofosbuvir; Velpatasvir, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
| Epclusa | US10086011 | Combination formulation of two antiviral compounds | Jan 30, 2034 | Gilead Sciences Inc |
| Epclusa |
US10086011 (Pediatric) | Combination formulation of two antiviral compounds | Jul 30, 2034 | Gilead Sciences Inc |
| Epclusa | US11116783 | Combination formulation of two antiviral compounds | Jan 30, 2034 | Gilead Sciences Inc |
| Epclusa |
US11116783 (Pediatric) | Combination formulation of two antiviral compounds | Jul 30, 2034 | Gilead Sciences Inc |
| Epclusa | US11707479 | Combination formulation of two antiviral compounds | Jan 30, 2034 | Gilead Sciences Inc |
| Epclusa |
US11707479 (Pediatric) | Combination formulation of two antiviral compounds | Jul 30, 2034 | Gilead Sciences Inc |
| Epclusa | US7964580 | NA | Mar 26, 2029 | Gilead Sciences Inc |
| Epclusa |
US7964580 (Pediatric) | NA | Sep 26, 2029 | Gilead Sciences Inc |
| Epclusa | US8334270 | NA | Mar 21, 2028 | Gilead Sciences Inc |
| Epclusa |
US8334270 (Pediatric) | NA | Sep 21, 2028 | Gilead Sciences Inc |
| Epclusa | US8575135 | Antiviral compounds | Nov 16, 2032 | Gilead Sciences Inc |
| Epclusa |
US8575135 (Pediatric) | Antiviral compounds | May 16, 2033 | Gilead Sciences Inc |
| Epclusa | US8580765 | NA | Mar 21, 2028 | Gilead Sciences Inc |
| Epclusa |
US8580765 (Pediatric) | NA | Sep 21, 2028 | Gilead Sciences Inc |
| Epclusa | US8618076 | Nucleoside phosphoramidates | Dec 11, 2030 | Gilead Sciences Inc |
| Epclusa |
US8618076 (Pediatric) | Nucleoside phosphoramidates | Jun 11, 2031 | Gilead Sciences Inc |
| Epclusa | US8633309 | Nucleoside phosphoramidates | Mar 26, 2029 | Gilead Sciences Inc |
| Epclusa |
US8633309 (Pediatric) | Nucleoside phosphoramidates | Sep 26, 2029 | Gilead Sciences Inc |
| Epclusa | US8735372 | NA | Mar 21, 2028 | Gilead Sciences Inc |
| Epclusa |
US8735372 (Pediatric) | NA | Sep 21, 2028 | Gilead Sciences Inc |
| Epclusa | US8889159 | Compositions and methods for treating hepatitis C virus | Mar 26, 2029 | Gilead Sciences Inc |
| Epclusa |
US8889159 (Pediatric) | Compositions and methods for treating hepatitis C virus | Sep 26, 2029 | Gilead Sciences Inc |
| Epclusa | US8921341 | Antiviral compounds | Nov 16, 2032 | Gilead Sciences Inc |
| Epclusa |
US8921341 (Pediatric) | Antiviral compounds | May 16, 2033 | Gilead Sciences Inc |
| Epclusa | US8940718 | Antiviral compounds | Nov 16, 2032 | Gilead Sciences Inc |
| Epclusa |
US8940718 (Pediatric) | Antiviral compounds | May 16, 2033 | Gilead Sciences Inc |
| Epclusa | US9085573 | NA | Mar 21, 2028 | Gilead Sciences Inc |
| Epclusa |
US9085573 (Pediatric) | NA | Sep 21, 2028 | Gilead Sciences Inc |
| Epclusa | US9284342 | Nucleoside phosphoramidates | Sep 13, 2030 | Gilead Sciences Inc |
| Epclusa |
US9284342 (Pediatric) | Nucleoside phosphoramidates | Mar 13, 2031 | Gilead Sciences Inc |
| Epclusa | US9757406 | Combination formulation of two antiviral compounds | Jan 30, 2034 | Gilead Sciences Inc |
| Epclusa |
US9757406 (Pediatric) | Combination formulation of two antiviral compounds | Jul 30, 2034 | Gilead Sciences Inc |
Coming Soon
Patent Strength Analyzer
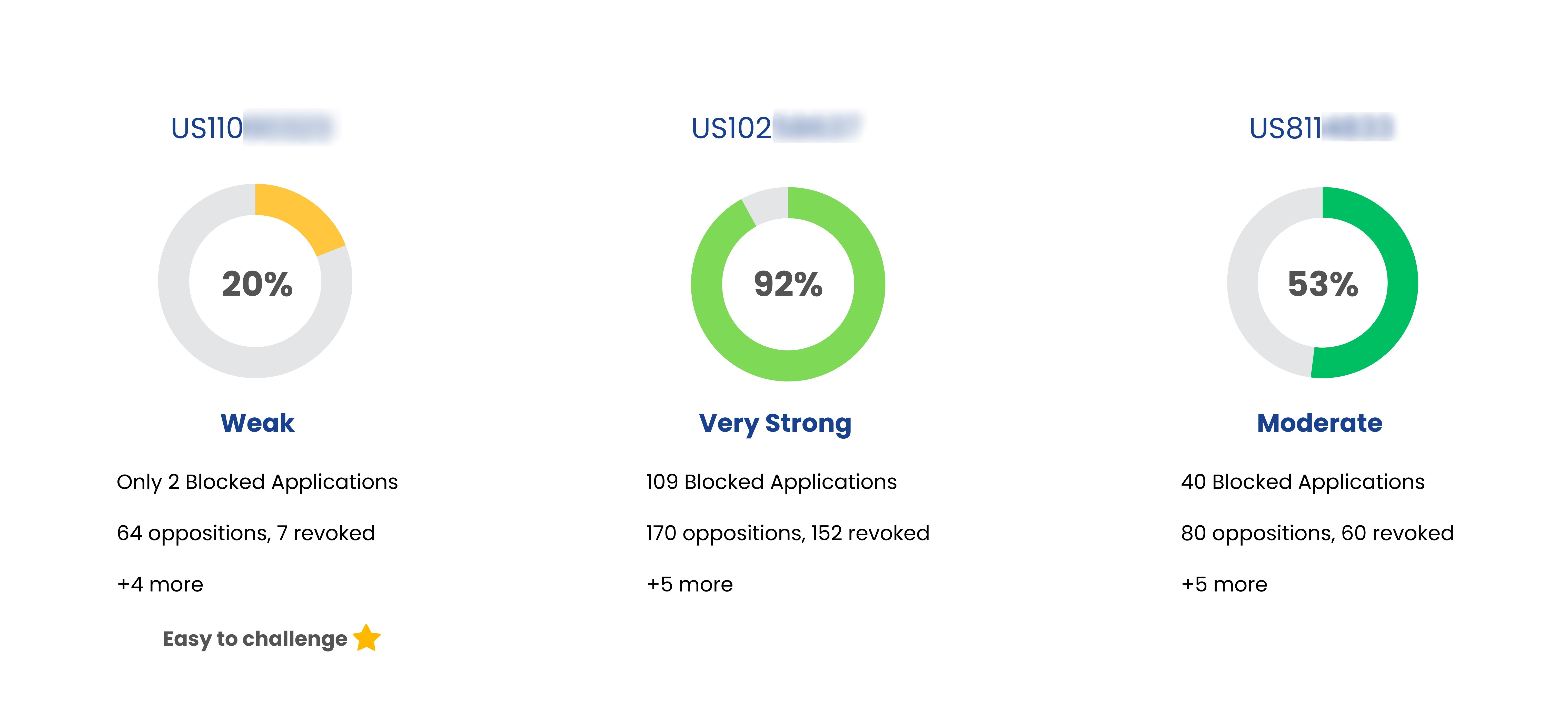
YesNo
Thank you for your response 🥳
