Naproxen Sodium; Sumatriptan Succinate Patent Expiration
Naproxen Sodium; Sumatriptan Succinate is Used for the treatment of migraines. It was first introduced by Currax Pharmaceuticals Llc
Naproxen Sodium; Sumatriptan Succinate Patents
Given below is the list of patents protecting Naproxen Sodium; Sumatriptan Succinate, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
| Treximet | US5872145 | Formulation of 5-HT agonist and NSAID for treatment of migraine |
Aug 14, 2017
(Expired) | Currax |
| Treximet |
US5872145 (Pediatric) | Formulation of 5-HT agonist and NSAID for treatment of migraine |
Feb 14, 2018
(Expired) | Currax |
| Treximet | US6060499 | Anti-migraine methods and compositions using 5-HT agonists with long-acting NSAIDs |
Aug 14, 2017
(Expired) | Currax |
| Treximet |
US6060499 (Pediatric) | Anti-migraine methods and compositions using 5-HT agonists with long-acting NSAIDs |
Feb 14, 2018
(Expired) | Currax |
| Treximet | US6586458 | Methods of treating headaches using 5-HT agonists in combination with long-acting NSAIDs |
Aug 14, 2017
(Expired) | Currax |
| Treximet |
US6586458 (Pediatric) | Methods of treating headaches using 5-HT agonists in combination with long-acting NSAIDs |
Feb 14, 2018
(Expired) | Currax |
| Treximet | US7332183 | Multilayer dosage forms containing NSAIDs and triptans | Oct 02, 2025 | Currax |
| Treximet |
US7332183 (Pediatric) | Multilayer dosage forms containing NSAIDs and triptans | Apr 02, 2026 | Currax |
| Treximet | US8022095 | Methods of treating headaches using 5-HT agonists in combination with long-acting NSAIDs |
Aug 14, 2017
(Expired) | Currax |
| Treximet |
US8022095 (Pediatric) | Methods of treating headaches using 5-HT agonists in combination with long-acting NSAIDs |
Feb 14, 2018
(Expired) | Currax |
A patent's expiry date may change depending upon legal activities going on that patent. Critical activities like abandoning of a patent, term extension of a patent or amendment of its claims can increase or decrease the life of a patent hence affecting its expiry date and in turn affecting the generic launch date of that drug. Tracking these ongoing activities on a patent application helps to keep an eye on the latest developments in the patent process of the drug which can give an idea of how early a drug's generic could be available. The next section provides a list of recent legal activities on Naproxen Sodium; Sumatriptan Succinate's patents.
Latest Legal Activities on Naproxen Sodium; Sumatriptan Succinate's Patents
Given below is the list recent legal activities going on the following patents of Naproxen Sodium; Sumatriptan Succinate.
| Event | Date | Patent/Publication |
|---|---|---|
 | ||
| Expire Patent | 28 Oct, 2019 | US8022095 |
| Payment of Maintenance Fee, 12th Year, Large Entity | 19 Aug, 2019 | US7332183 |
| Maintenance Fee Reminder Mailed | 13 May, 2019 | US8022095 |
| Request for Trial Denied | 06 May, 2016 | US7332183 |
| Petition Requesting Trial | 12 Nov, 2015 | US7332183 |
| Email Notification | 04 Sep, 2012 | US7332183 |
| Change in Power of Attorney (May Include Associate POA) | 04 Sep, 2012 | US7332183 |
| Correspondence Address Change | 01 Sep, 2012 | US7332183 |
| Email Notification | 21 Sep, 2011 | US8022095 |
| Recordation of Patent Grant Mailed | 20 Sep, 2011 | US8022095 |
Coming Soon
Patent Strength Analyzer
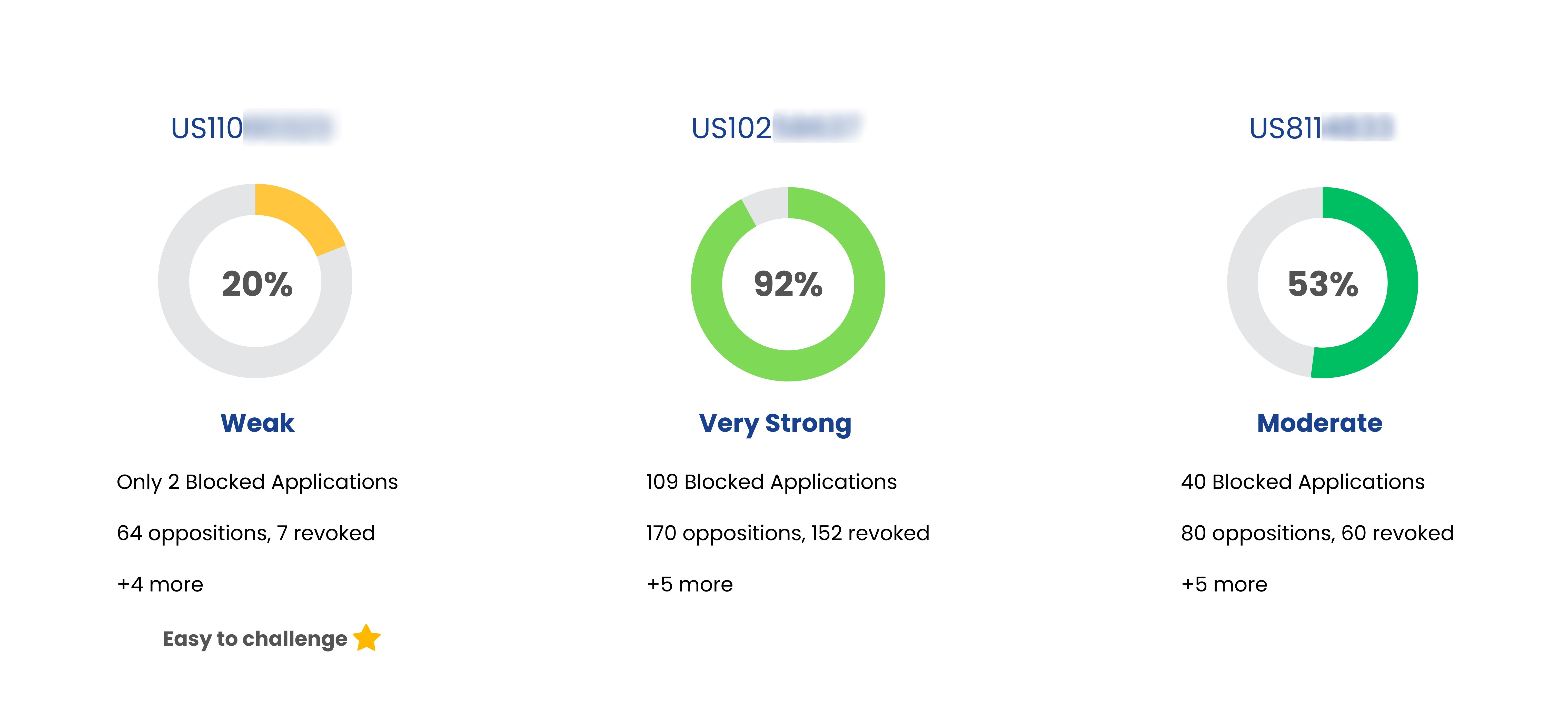
YesNo
Thank you for your response 🥳
Naproxen Sodium; Sumatriptan Succinate Generics
Several generic applications have been filed for Naproxen Sodium; Sumatriptan Succinate. The first generic version for Naproxen Sodium; Sumatriptan Succinate was by Aurobindo Pharma Ltd and was approved on Feb 15, 2018. And the latest generic version is by Rising Pharma Holdings Inc and was approved on Sep 4, 2018.
Given below is the list of companies who have filed for Naproxen Sodium; Sumatriptan Succinate generic.
1. SUN PHARM
Sun Pharmaceutical Industries Ltd has filed for 1 generic for Naproxen Sodium; Sumatriptan Succinate. This 500mg;eq 85mg base version comes by the name SUMATRIPTAN AND NAPROXEN SODIUM. Given below are the details of the strengths of this generic introduced by Sun Pharm.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 500MG; EQ 85MG BASE | tablet | Prescription | ORAL | AB | Jul 20, 2018 |
2. AUROBINDO PHARMA LTD
Aurobindo Pharma Ltd has filed for 1 generic for Naproxen Sodium; Sumatriptan Succinate. This 500mg;eq 85mg base version comes by the name SUMATRIPTAN AND NAPROXEN SODIUM. Given below are the details of the strengths of this generic introduced by Aurobindo Pharma Ltd.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 500MG; EQ 85MG BASE | tablet | Prescription | ORAL | AB | Feb 15, 2018 |
3. RISING
Rising Pharma Holdings Inc has filed for 1 generic for Naproxen Sodium; Sumatriptan Succinate. This 500mg;eq 85mg base version comes by the name SUMATRIPTAN AND NAPROXEN SODIUM. Given below are the details of the strengths of this generic introduced by Rising.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 500MG; EQ 85MG BASE | tablet | Prescription | ORAL | AB | Sep 4, 2018 |
