Linagliptin; Metformin Hydrochloride Patent Expiration
Linagliptin; Metformin Hydrochloride is used for managing type 2 diabetes with renal impairment and inadequate glycemic control. It was first introduced by Boehringer Ingelheim Pharmaceuticals Inc
Linagliptin; Metformin Hydrochloride Patents
Given below is the list of patents protecting Linagliptin; Metformin Hydrochloride, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
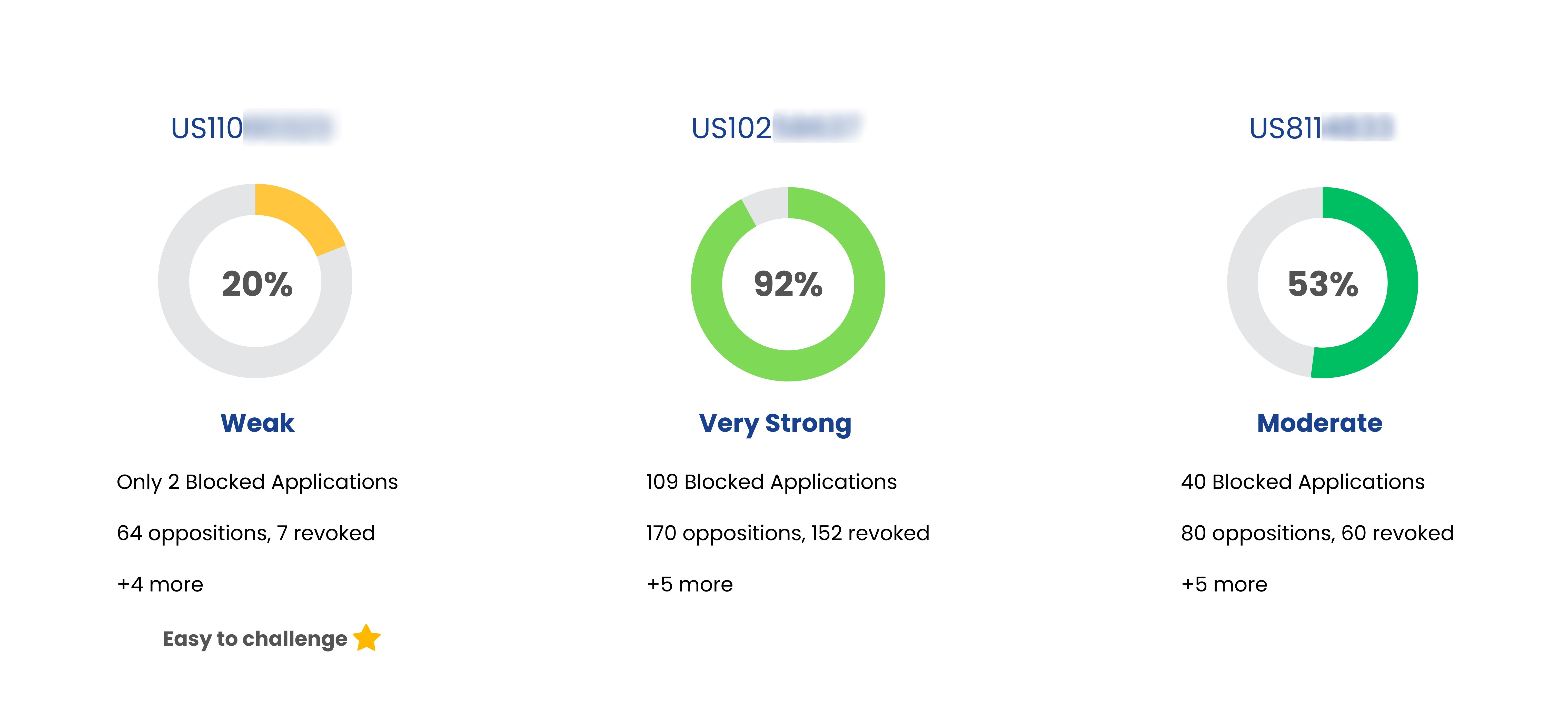
| Jentadueto | US10022379 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Apr 02, 2029 | Boehringer Ingelheim |
| Jentadueto |
US10022379 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Oct 02, 2029 | Boehringer Ingelheim |
| Jentadueto | US10973827 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Apr 02, 2029 | Boehringer Ingelheim |
| Jentadueto |
US10973827 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Oct 02, 2029 | Boehringer Ingelheim |
| Jentadueto | US11911388 | Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral or non-oral antidiabetic drug | Apr 10, 2030 | Boehringer Ingelheim |
| Jentadueto | US6303661 | Use of dipeptidyl peptidase IV effectors for lowering the blood glucose level in mammals |
Apr 24, 2017
(Expired) | Boehringer Ingelheim |
| Jentadueto | US6890898 | Method of regulating glucose metabolism, and reagents related thereto |
Feb 02, 2019
(Expired) | Boehringer Ingelheim |
| Jentadueto | US7078381 | Method of regulating glucose metabolism, and reagents related thereto |
Feb 02, 2019
(Expired) | Boehringer Ingelheim |
| Jentadueto | US7407955 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions | May 02, 2025 | Boehringer Ingelheim |
| Jentadueto |
US7407955 (Pediatric) | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions | Nov 02, 2025 | Boehringer Ingelheim |
| Jentadueto | US7459428 | Method of regulating glucose metabolism, and reagents related thereto |
Feb 02, 2019
(Expired) | Boehringer Ingelheim |
| Jentadueto | US8119648 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
Aug 12, 2023
(Expired) | Boehringer Ingelheim |
| Jentadueto | US8178541 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
Aug 12, 2023
(Expired) | Boehringer Ingelheim |
| Jentadueto | US8673927 | Uses of DPP-IV inhibitors | May 04, 2027 | Boehringer Ingelheim |
| Jentadueto |
US8673927 (Pediatric) | Uses of DPP-IV inhibitors | Nov 04, 2027 | Boehringer Ingelheim |
| Jentadueto | US8846695 | Treatment for diabetes in patients with inadequate glycemic control despite metformin therapy comprising a DPP-IV inhibitor | Jun 04, 2030 | Boehringer Ingelheim |
| Jentadueto |
US8846695 (Pediatric) | Treatment for diabetes in patients with inadequate glycemic control despite metformin therapy comprising a DPP-IV inhibitor | Dec 04, 2030 | Boehringer Ingelheim |
| Jentadueto | US8883805 | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines | Nov 26, 2025 | Boehringer Ingelheim |
| Jentadueto |
US8883805 (Pediatric) | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines | May 26, 2026 | Boehringer Ingelheim |
| Jentadueto | US9155705 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | May 21, 2030 | Boehringer Ingelheim |
| Jentadueto |
US9155705 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Nov 21, 2030 | Boehringer Ingelheim |
| Jentadueto | US9173859 | Uses of DPP IV inhibitors | May 04, 2027 | Boehringer Ingelheim |
| Jentadueto | US9415016 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Apr 02, 2029 | Boehringer Ingelheim |
| Jentadueto |
US9415016 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Oct 02, 2029 | Boehringer Ingelheim |
| Jentadueto Xr | US10022379 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Apr 02, 2029 | Boehringer Ingelheim |
| Jentadueto Xr |
US10022379 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Oct 02, 2029 | Boehringer Ingelheim |
| Jentadueto Xr | US11911388 | Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral or non-oral antidiabetic drug | Apr 10, 2030 | Boehringer Ingelheim |
| Jentadueto Xr | US6303661 | Use of dipeptidyl peptidase IV effectors for lowering the blood glucose level in mammals |
Apr 24, 2017
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US6340475 | Extending the duration of drug release within the stomach during the fed mode |
Sep 19, 2016
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US6488962 | Tablet shapes to enhance gastric retention of swellable controlled-release oral dosage forms |
Jun 20, 2020
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US6635280 | Extending the duration of drug release within the stomach during the fed mode |
Sep 19, 2016
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US6890898 | Method of regulating glucose metabolism, and reagents related thereto |
Feb 02, 2019
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US7078381 | Method of regulating glucose metabolism, and reagents related thereto |
Feb 02, 2019
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US7407955 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions | May 02, 2025 | Boehringer Ingelheim |
| Jentadueto Xr |
US7407955 (Pediatric) | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions | Nov 02, 2025 | Boehringer Ingelheim |
| Jentadueto Xr | US7459428 | Method of regulating glucose metabolism, and reagents related thereto |
Feb 02, 2019
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US8119648 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
Aug 12, 2023
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US8178541 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
Aug 12, 2023
(Expired) | Boehringer Ingelheim |
| Jentadueto Xr | US8673927 | Uses of DPP-IV inhibitors | May 04, 2027 | Boehringer Ingelheim |
| Jentadueto Xr |
US8673927 (Pediatric) | Uses of DPP-IV inhibitors | Nov 04, 2027 | Boehringer Ingelheim |
| Jentadueto Xr | US8846695 | Treatment for diabetes in patients with inadequate glycemic control despite metformin therapy comprising a DPP-IV inhibitor | Jun 04, 2030 | Boehringer Ingelheim |
| Jentadueto Xr | US8883805 | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines | Nov 26, 2025 | Boehringer Ingelheim |
| Jentadueto Xr |
US8883805 (Pediatric) | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines | May 26, 2026 | Boehringer Ingelheim |
| Jentadueto Xr | US9155705 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | May 21, 2030 | Boehringer Ingelheim |
| Jentadueto Xr |
US9155705 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Nov 21, 2030 | Boehringer Ingelheim |
| Jentadueto Xr | US9173859 | Uses of DPP IV inhibitors | May 04, 2027 | Boehringer Ingelheim |
| Jentadueto Xr |
US9173859 (Pediatric) | Uses of DPP IV inhibitors | Nov 04, 2027 | Boehringer Ingelheim |
| Jentadueto Xr | US9415016 | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Apr 02, 2029 | Boehringer Ingelheim |
| Jentadueto Xr |
US9415016 (Pediatric) | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation | Oct 02, 2029 | Boehringer Ingelheim |
| Jentadueto Xr | US9555001 | Pharmaceutical composition and uses thereof | Mar 06, 2033 | Boehringer Ingelheim |
| Jentadueto Xr |
US9555001 (Pediatric) | Pharmaceutical composition and uses thereof | Sep 06, 2033 | Boehringer Ingelheim |
Coming Soon
Patent Strength Analyzer
YesNo
Thank you for your response 🥳
Linagliptin; Metformin Hydrochloride Generics
Several generic applications have been filed for Linagliptin; Metformin Hydrochloride. The first generic version for Linagliptin; Metformin Hydrochloride was by Sunshine Lake Pharma Co Ltd and was approved on Aug 30, 2021. And the latest generic version is by Zydus Pharmaceuticals Usa Inc and was approved on Mar 30, 2023.
Given below is the list of companies who have filed for Linagliptin; Metformin Hydrochloride generic.
1. SUNSHINE
Sunshine Lake Pharma Co Ltd has filed for 3 different strengths of generic version for Linagliptin; Metformin Hydrochloride. All of these versions come by the name LINAGLIPTIN AND METFORMIN HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Sunshine.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 2.5MG; 500MG | tablet | Prescription | ORAL | AB | Aug 30, 2021 |
| 2.5MG; 850MG | tablet | Prescription | ORAL | AB | Aug 30, 2021 |
| 2.5MG; 1GM | tablet | Prescription | ORAL | AB | Aug 30, 2021 |
2. ZYDUS PHARMS
Zydus Pharmaceuticals Usa Inc has filed for 3 different strengths of generic version for Linagliptin; Metformin Hydrochloride. All of these versions come by the name LINAGLIPTIN AND METFORMIN HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Zydus Pharms.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 2.5MG; 500MG | tablet | Discontinued | ORAL | N/A | Mar 30, 2023 |
| 2.5MG; 850MG | tablet | Discontinued | ORAL | N/A | Mar 30, 2023 |
| 2.5MG; 1GM | tablet | Discontinued | ORAL | N/A | Mar 30, 2023 |
