Lidocaine; Tetracaine Patent Expiration
Lidocaine; Tetracaine was first introduced by Galen Specialty Pharma Us Llc
Lidocaine; Tetracaine Patents
Given below is the list of patents protecting Lidocaine; Tetracaine, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
| Pliaglis | US10350180 | Solid-forming local anesthetic formulations for pain control | Jan 14, 2031 | Taro |
| Pliaglis | US10603293 | Solid-forming local anesthetic formulations for pain control | Jan 14, 2031 | Taro |
| Pliaglis | US10751305 | Solid-forming topical formulations for pain control | Jan 14, 2031 | Taro |
| Pliaglis | US5919479 | Noninvasive dermal anesthetics |
Jul 28, 2015
(Expired) | Taro |
| Pliaglis | US6528086 | Methods and apparatus for drug delivery involving phase changing formulations |
Sep 28, 2019
(Expired) | Taro |
| Synera | US5658583 | Apparatus and methods for improved noninvasive dermal administration of pharmaceuticals |
Jul 28, 2015
(Expired) | Galen Specialty |
| Synera | US5919479 | Noninvasive dermal anesthetics |
Jul 28, 2015
(Expired) | Galen Specialty |
| Synera | US6306431 | Apparatus for heating to a desired temperature for improved administration of pharmaceutically active compounds |
Jul 28, 2015
(Expired) | Galen Specialty |
| Synera | US6465006 | Method for facilitating absorption of pharmaceutically active compounds |
Jul 28, 2015
(Expired) | Galen Specialty |
| Synera | US6465709 | Exothermic bandage |
Jul 07, 2020
(Expired) | Galen Specialty |
| Synera | US6546281 | Integrated apparatus for controlled heat aided dermal drug delivery |
Jul 28, 2015
(Expired) | Galen Specialty |
| Synera | US6780426 | Method and apparatus for improved heat controlled administration of pharmaceuticals |
Jul 28, 2015
(Expired) | Galen Specialty |
A patent's expiry date may change depending upon legal activities going on that patent. Critical activities like abandoning of a patent, term extension of a patent or amendment of its claims can increase or decrease the life of a patent hence affecting its expiry date and in turn affecting the generic launch date of that drug. Tracking these ongoing activities on a patent application helps to keep an eye on the latest developments in the patent process of the drug which can give an idea of how early a drug's generic could be available. The next section provides a list of recent legal activities on Lidocaine; Tetracaine's patents.
Latest Legal Activities on Lidocaine; Tetracaine's Patents
Given below is the list recent legal activities going on the following patents of Lidocaine; Tetracaine.
| Event | Date | Patent/Publication |
|---|---|---|
 | ||
| Expire Patent | 16 Sep, 2016 | US6780426 |
| Entity Status Set To Undiscounted (Initial Default Setting or Status Change) | 04 Mar, 2008 | US6780426 |
| Recordation of Patent Grant Mailed | 24 Aug, 2004 | US6780426 |
| Patent Issue Date Used in PTA Calculation | 24 Aug, 2004 | US6780426 |
| Issue Notification Mailed | 05 Aug, 2004 | US6780426 |
| Receipt into Pubs | 23 Jul, 2004 | US6780426 |
| Dispatch to FDC | 21 Jul, 2004 | US6780426 |
| Application Is Considered Ready for Issue | 02 Jul, 2004 | US6780426 |
| Issue Fee Payment Received | 14 Jun, 2004 | US6780426 |
| Issue Fee Payment Verified | 14 Jun, 2004 | US6780426 |
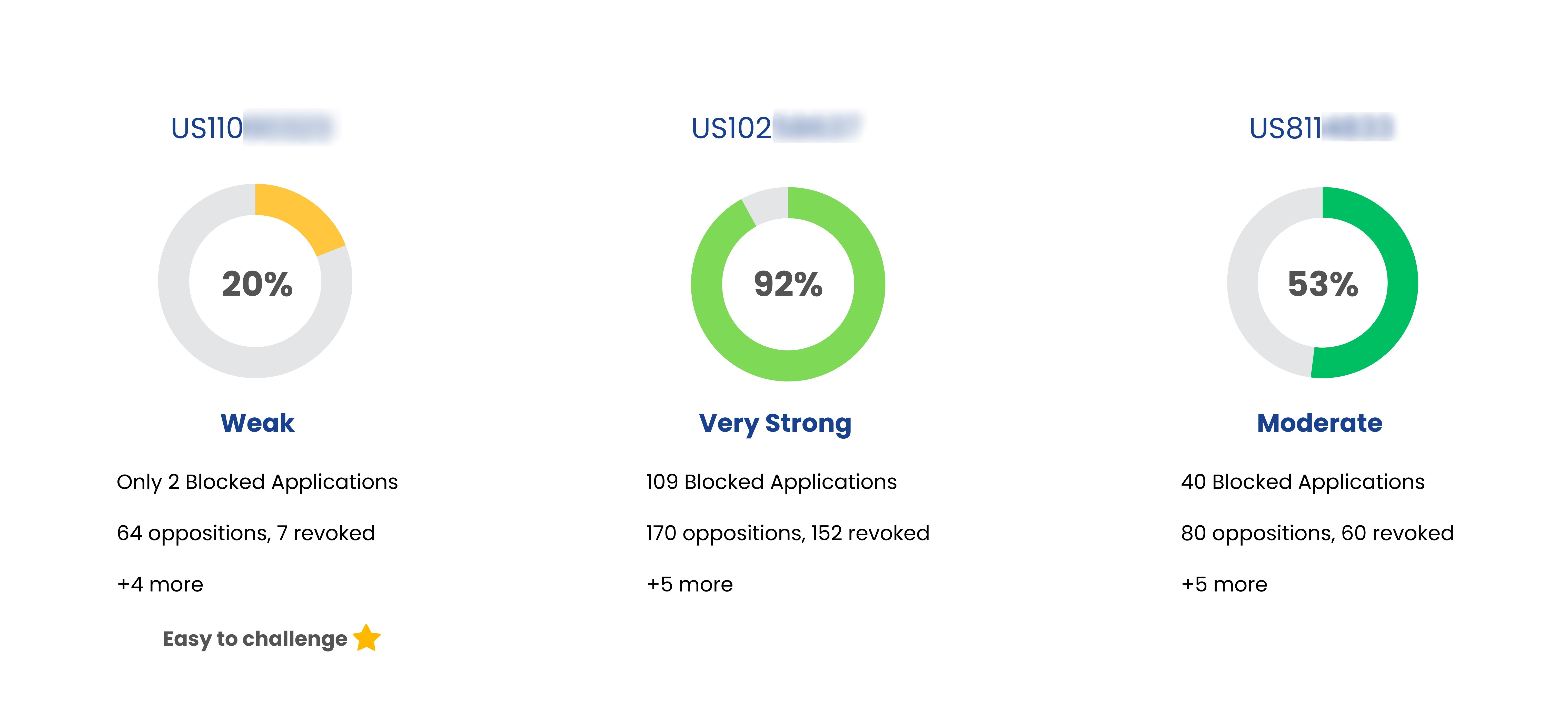
Coming Soon
Patent Strength Analyzer
YesNo
Thank you for your response 🥳
