Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride Patent Expiration
Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride is Used for relieving symptoms of seasonal and perennial allergic rhinitis. It was first introduced by Johnson And Johnson Consumer Inc Mcneil Consumer Healthcare Div
Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride Patents
Given below is the list of patents protecting Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
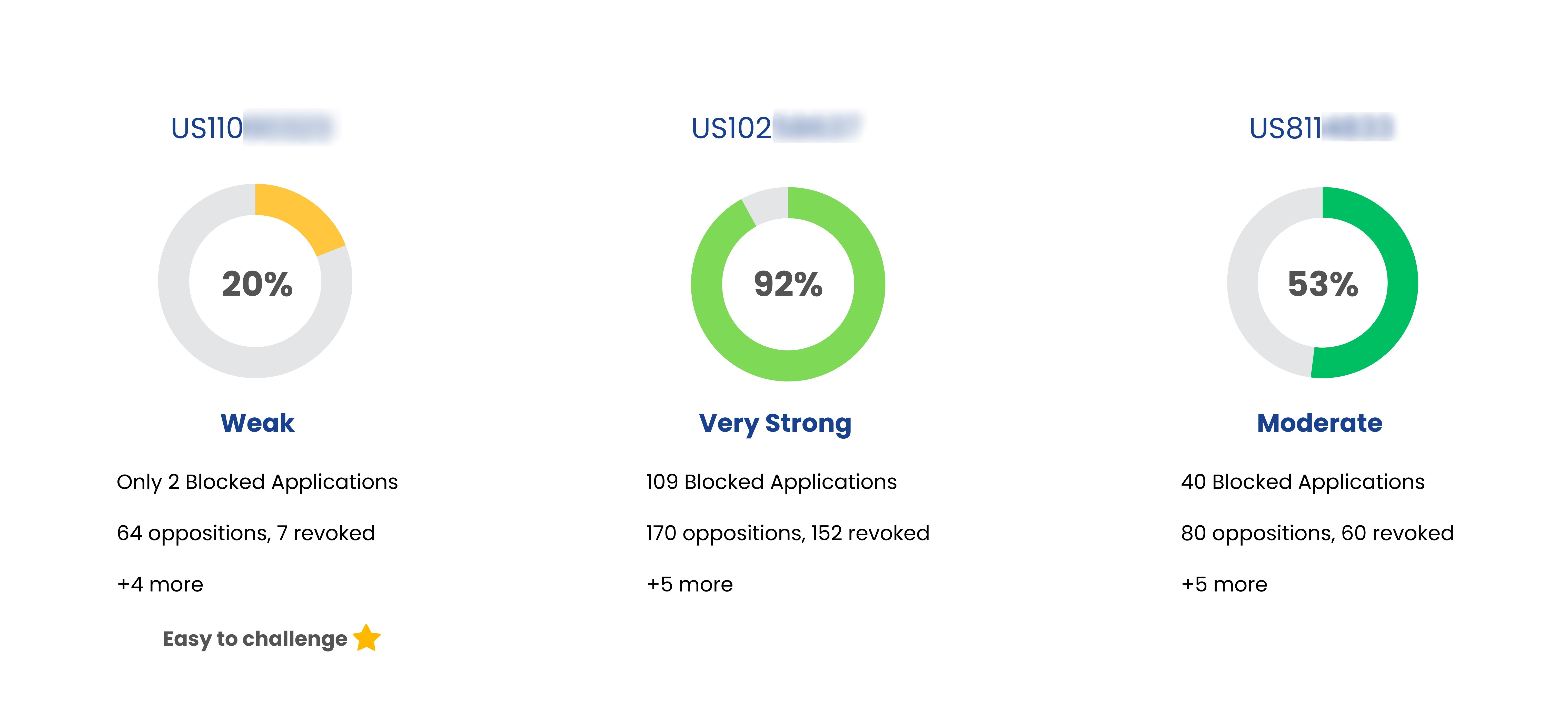
| Zyrtec-d 12 Hour | US6469009 | Pharmaceutical compositions for the treatment of rhinitis |
Jul 13, 2019
(Expired) | J And J Consumer Inc |
| Zyrtec-d 12 Hour | US6489329 | Pharmaceutical compositions for the treatment of rhinitis |
Apr 08, 2016
(Expired) | J And J Consumer Inc |
| Zyrtec-d 12 Hour | US7014867 | Tablet comprising cetirizine and pseudoephedrine |
Jun 10, 2022
(Expired) | J And J Consumer Inc |
| Zyrtec-d 12 Hour | US7226614 | Tablet comprising cetirizine and pseudoephedrine |
Jun 10, 2022
(Expired) | J And J Consumer Inc |
A patent's expiry date may change depending upon legal activities going on that patent. Critical activities like abandoning of a patent, term extension of a patent or amendment of its claims can increase or decrease the life of a patent hence affecting its expiry date and in turn affecting the generic launch date of that drug. Tracking these ongoing activities on a patent application helps to keep an eye on the latest developments in the patent process of the drug which can give an idea of how early a drug's generic could be available. The next section provides a list of recent legal activities on Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride's patents.
Latest Legal Activities on Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride's Patents
Given below is the list recent legal activities going on the following patents of Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride.
| Event | Date | Patent/Publication |
|---|---|---|
 | ||
| Payment of Maintenance Fee, 12th Year, Large Entity | 22 Nov, 2018 | US7226614 |
| Payment of Maintenance Fee, 12th Year, Large Entity | 07 Sep, 2017 | US7014867(Litigated) |
| Recordation of Patent Grant Mailed | 05 Jun, 2007 | US7226614 |
| Patent Issue Date Used in PTA Calculation | 05 Jun, 2007 | US7226614 |
| Issue Notification Mailed | 16 May, 2007 | US7226614 |
| Dispatch to FDC | 07 May, 2007 | US7226614 |
| Application Is Considered Ready for Issue | 01 May, 2007 | US7226614 |
| Issue Fee Payment Received | 25 Apr, 2007 | US7226614 |
| Issue Fee Payment Verified | 25 Apr, 2007 | US7226614 |
| Mail Notice of Allowance | 06 Apr, 2007 | US7226614 |
Coming Soon
Patent Strength Analyzer
YesNo
Thank you for your response 🥳
Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride Generics
Several generic applications have been filed for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride. The first generic version for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride was by Ivax Pharmaceuticals Inc Sub Teva Pharmaceuticals Usa and was approved on Feb 25, 2008. And the latest generic version is by Aurobindo Pharma Ltd and was approved on Mar 8, 2023.
Given below is the list of companies who have filed for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride generic.
1. PPI-DAC
Perrigo Pharma International Dac has filed for 1 generic for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride. This 5mg;120mg version comes by the name CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Ppi-dac.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 5MG; 120MG | tablet, extended release | Over the counter | ORAL | N/A | Nov 16, 2018 |
2. AUROBINDO PHARMA LTD
Aurobindo Pharma Ltd has filed for 1 generic for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride. This 5mg;120mg version comes by the name CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Aurobindo Pharma Ltd.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 5MG; 120MG | tablet, extended release | Discontinued | ORAL | N/A | Mar 8, 2023 |
3. SUN PHARM INDS LTD
Sun Pharmaceutical Industries Ltd has filed for 1 generic for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride. This 5mg;120mg version comes by the name CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Sun Pharm Inds Ltd.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 5MG; 120MG | tablet, extended release | Over the counter | ORAL | N/A | Sep 28, 2012 |
4. IVAX SUB TEVA PHARMS
Ivax Pharmaceuticals Inc Sub Teva Pharmaceuticals Usa has filed for 1 generic for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride. This 5mg;120mg version comes by the name CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Ivax Sub Teva Pharms.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 5MG; 120MG | tablet, extended release | Over the counter | ORAL | N/A | Feb 25, 2008 |
5. PLD ACQUISITIONS
Pld Acquisitions Llc Dba Avema Pharma Solutions has filed for 1 generic for Cetirizine Hydrochloride; Pseudoephedrine Hydrochloride. This 5mg;120mg version comes by the name CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE. Given below are the details of the strengths of this generic introduced by Pld Acquisitions.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 5MG; 120MG | tablet, extended release | Over the counter | ORAL | N/A | Mar 5, 2008 |
