Budesonide; Formoterol Fumarate Dihydrate Patent Expiration
Budesonide; Formoterol Fumarate Dihydrate is Used for managing and treating chronic respiratory conditions like COPD and asthma. It was first introduced by Astrazeneca Lp
Budesonide; Formoterol Fumarate Dihydrate Patents
Given below is the list of patents protecting Budesonide; Formoterol Fumarate Dihydrate, along with the drug name that holds that patent and the company name owning that drug.
| Drug Used in | Drug Patent Number | Drug Patent Title | Drug Patent Expiry | Drug Owner |
|---|---|---|---|---|
| Symbicort | US10166247 | Composition for inhalation |
Jan 29, 2023
(Expired) | Astrazeneca |
| Symbicort |
US10166247 (Pediatric) | Composition for inhalation |
Jul 29, 2023
(Expired) | Astrazeneca |
| Symbicort | US11311558 | Composition for inhalation |
Jan 29, 2023
(Expired) | Astrazeneca |
| Symbicort |
US11311558 (Pediatric) | Composition for inhalation |
Jul 29, 2023
(Expired) | Astrazeneca |
| Symbicort | US5674860 | Combination of a bronchodilator and a steroidal anti-inflammatory drug for the treatment of respiratory disorders |
Oct 07, 2014
(Expired) | Astrazeneca |
| Symbicort | US5972919 | Combination of a bronchodilator and a steroidal anti-inflammatory drug for the treatment of respiratory disorders, as well as its use and the preparation thereof |
Dec 17, 2012
(Expired) | Astrazeneca |
| Symbicort | US6123924 | Pressurized aerosol inhalation compositions |
Sep 26, 2017
(Expired) | Astrazeneca |
| Symbicort |
US6123924 (Pediatric) | Pressurized aerosol inhalation compositions |
Mar 26, 2018
(Expired) | Astrazeneca |
| Symbicort | US6641800 | Pressurized aerosol compositions comprising powdered medicament dispersed in hydrofluoroalkane |
Sep 23, 2012
(Expired) | Astrazeneca |
| Symbicort | US7367333 | Inhalation device |
Nov 11, 2018
(Expired) | Astrazeneca |
| Symbicort |
US7367333 (Pediatric) | Inhalation device |
May 11, 2019
(Expired) | Astrazeneca |
| Symbicort | US7587988 | Inhaler device counter | Apr 10, 2026 | Astrazeneca |
| Symbicort |
US7587988 (Pediatric) | Inhaler device counter | Oct 10, 2026 | Astrazeneca |
| Symbicort | US7759328 | Composition for inhalation |
Jan 29, 2023
(Expired) | Astrazeneca |
| Symbicort |
US7759328 (Pediatric) | Composition for inhalation |
Jul 29, 2023
(Expired) | Astrazeneca |
| Symbicort | US7897646 | Use for budesonide and formoterol |
Sep 09, 2018
(Expired) | Astrazeneca |
| Symbicort |
US7897646 (Pediatric) | Use for budesonide and formoterol |
Mar 09, 2019
(Expired) | Astrazeneca |
| Symbicort | US7967011 | Inhalation device |
Aug 11, 2021
(Expired) | Astrazeneca |
| Symbicort |
US7967011 (Pediatric) | Inhalation device |
Feb 11, 2022
(Expired) | Astrazeneca |
| Symbicort | US8143239 | Composition for inhalation |
Jan 29, 2023
(Expired) | Astrazeneca |
| Symbicort |
US8143239 (Pediatric) | Composition for inhalation |
Jul 29, 2023
(Expired) | Astrazeneca |
| Symbicort | US8387615 | Inhaler cap strap | Mar 26, 2027 | Astrazeneca |
| Symbicort |
US8387615 (Pediatric) | Inhaler cap strap | Sep 26, 2027 | Astrazeneca |
| Symbicort | US8461211 | Use for budesonide and formoterol |
Sep 09, 2018
(Expired) | Astrazeneca |
| Symbicort |
US8461211 (Pediatric) | Use for budesonide and formoterol |
Mar 09, 2019
(Expired) | Astrazeneca |
| Symbicort | US8528545 | Inhaler device that reduces the risk for miscounting a dosage | Oct 16, 2028 | Astrazeneca |
| Symbicort |
US8528545 (Pediatric) | Inhaler device that reduces the risk for miscounting a dosage | Apr 16, 2029 | Astrazeneca |
| Symbicort | US8575137 | Composition for inhalation |
Jan 29, 2023
(Expired) | Astrazeneca |
| Symbicort |
US8575137 (Pediatric) | Composition for inhalation |
Jul 29, 2023
(Expired) | Astrazeneca |
| Symbicort | US8616196 | Inhalation device and a method for assembling said inhalation device | Apr 07, 2029 | Astrazeneca |
| Symbicort |
US8616196 (Pediatric) | Inhalation device and a method for assembling said inhalation device | Oct 07, 2029 | Astrazeneca |
| Symbicort | US8875699 | Inhaler cap strap | Nov 10, 2024 | Astrazeneca |
| Symbicort |
US8875699 (Pediatric) | Inhaler cap strap | May 10, 2025 | Astrazeneca |
A patent's expiry date may change depending upon legal activities going on that patent. Critical activities like abandoning of a patent, term extension of a patent or amendment of its claims can increase or decrease the life of a patent hence affecting its expiry date and in turn affecting the generic launch date of that drug. Tracking these ongoing activities on a patent application helps to keep an eye on the latest developments in the patent process of the drug which can give an idea of how early a drug's generic could be available. The next section provides a list of recent legal activities on Budesonide; Formoterol Fumarate Dihydrate's patents.
Latest Legal Activities on Budesonide; Formoterol Fumarate Dihydrate's Patents
Given below is the list recent legal activities going on the following patents of Budesonide; Formoterol Fumarate Dihydrate.
| Event | Date | Patent/Publication |
|---|---|---|
 | ||
| Expire Patent | 29 Apr, 2024 | US8143239(Litigated) |
| Maintenance Fee Reminder Mailed | 13 Nov, 2023 | US8143239(Litigated) |
| Expire Patent | 31 Jul, 2023 | US7967011 |
| Expire Patent | 03 Apr, 2023 | US7897646 |
| Maintenance Fee Reminder Mailed | 13 Feb, 2023 | US7967011 |
| Maintenance Fee Reminder Mailed | 17 Oct, 2022 | US7897646 |
| Payment of Maintenance Fee, 4th Year, Large Entity | 15 Jun, 2022 | US10166247(Litigated) |
| Recordation of Patent Grant Mailed | 26 Apr, 2022 | US11311558(Litigated) |
| Patent Issue Date Used in PTA Calculation | 26 Apr, 2022 | US11311558(Litigated) |
| Payment of Maintenance Fee, 8th Year, Large Entity | 20 Apr, 2022 | US8875699 |
Coming Soon
Patent Strength Analyzer
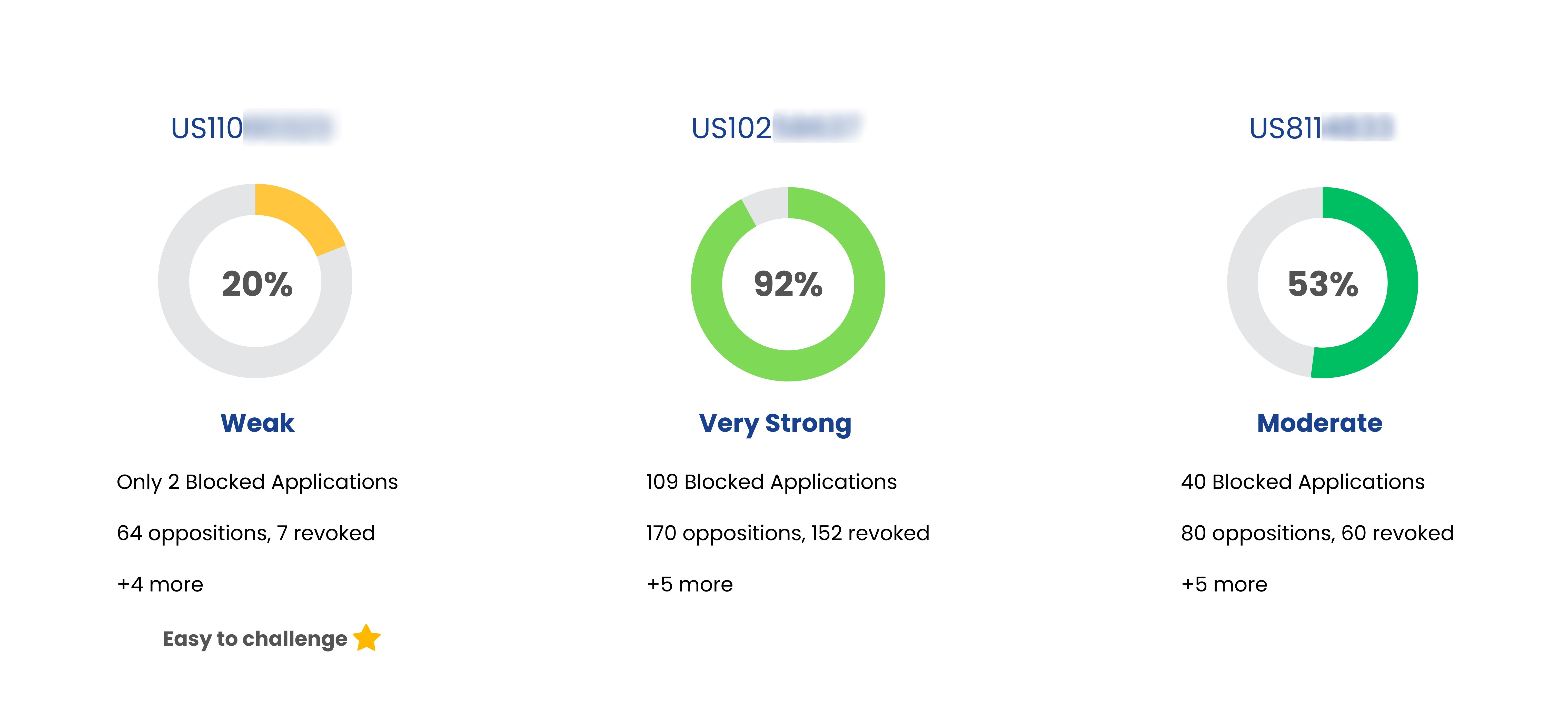
YesNo
Thank you for your response 🥳
Budesonide; Formoterol Fumarate Dihydrate Generics
Several generic applications have been filed for Budesonide; Formoterol Fumarate Dihydrate.
Given below is the list of companies who have filed for Budesonide; Formoterol Fumarate Dihydrate generic.
1. MYLAN
Mylan Pharmaceuticals Inc has filed for 2 different strengths of generic version for Budesonide; Formoterol Fumarate Dihydrate. All of these versions come by the name BREYNA. Given below are the details of the strengths of this generic introduced by Mylan.
| Strength | Dosage Form | Availability | Application Pathway | TE code | Launch Date |
|---|---|---|---|---|---|
| 0.08MG/INH; 0.0045MG/INH | aerosol, metered | Prescription | INHALATION | AB | Mar 15, 2022 |
| 0.16MG/INH; 0.0045MG/INH | aerosol, metered | Prescription | INHALATION | AB | Mar 15, 2022 |
